Student Exploration: Energy Conversion in a System
Vocabulary: energy, gravitational potential energy, heat energy, kinetic energy, law of conservation of energy, specific heat capacity
Prior Knowledge Questions (Do these BEFORE using the Gizmo.)
A battery contains stored energy in the form of chemical energy.
1. What are some examples of devices that are powered by batteries?
2. What different forms of energy are demonstrated by these devices?
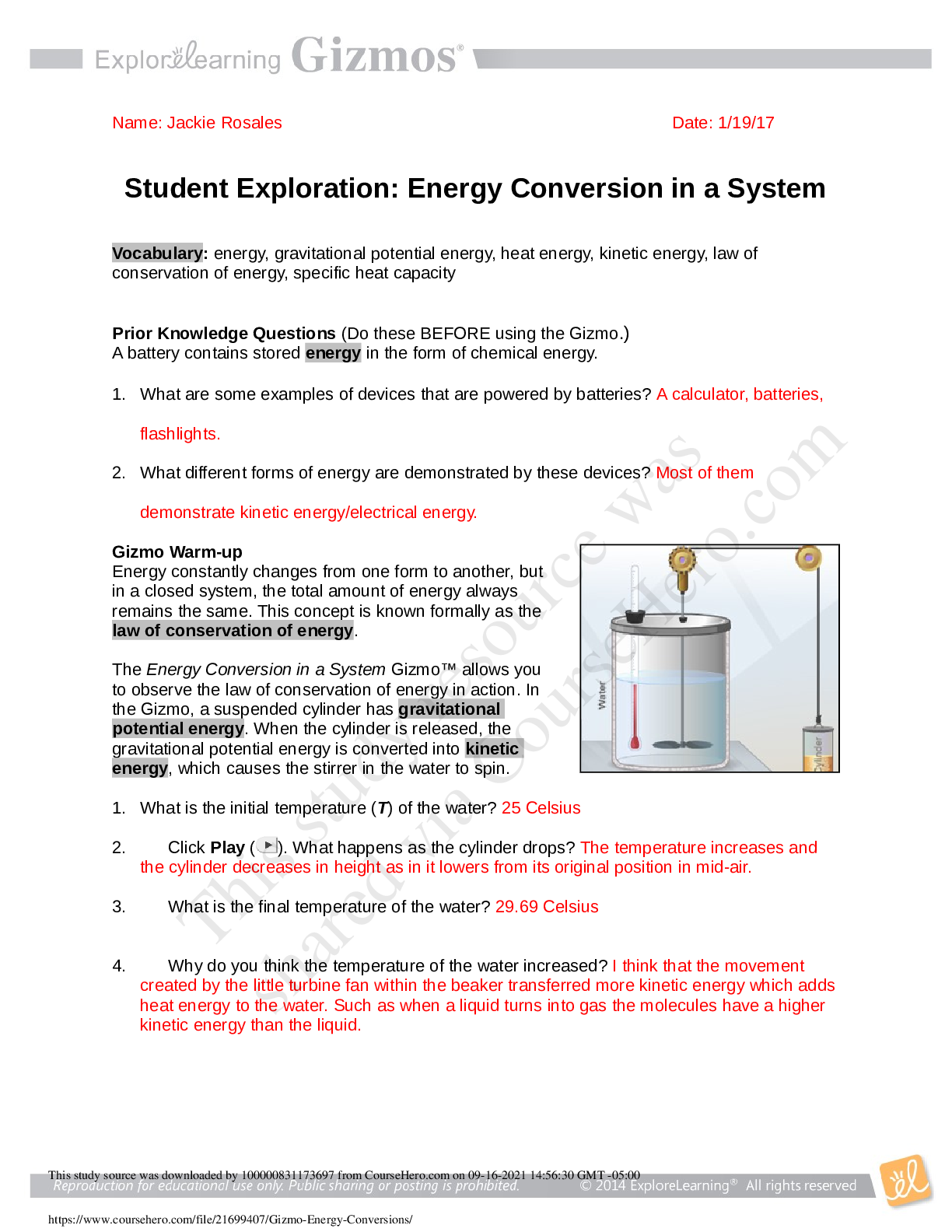
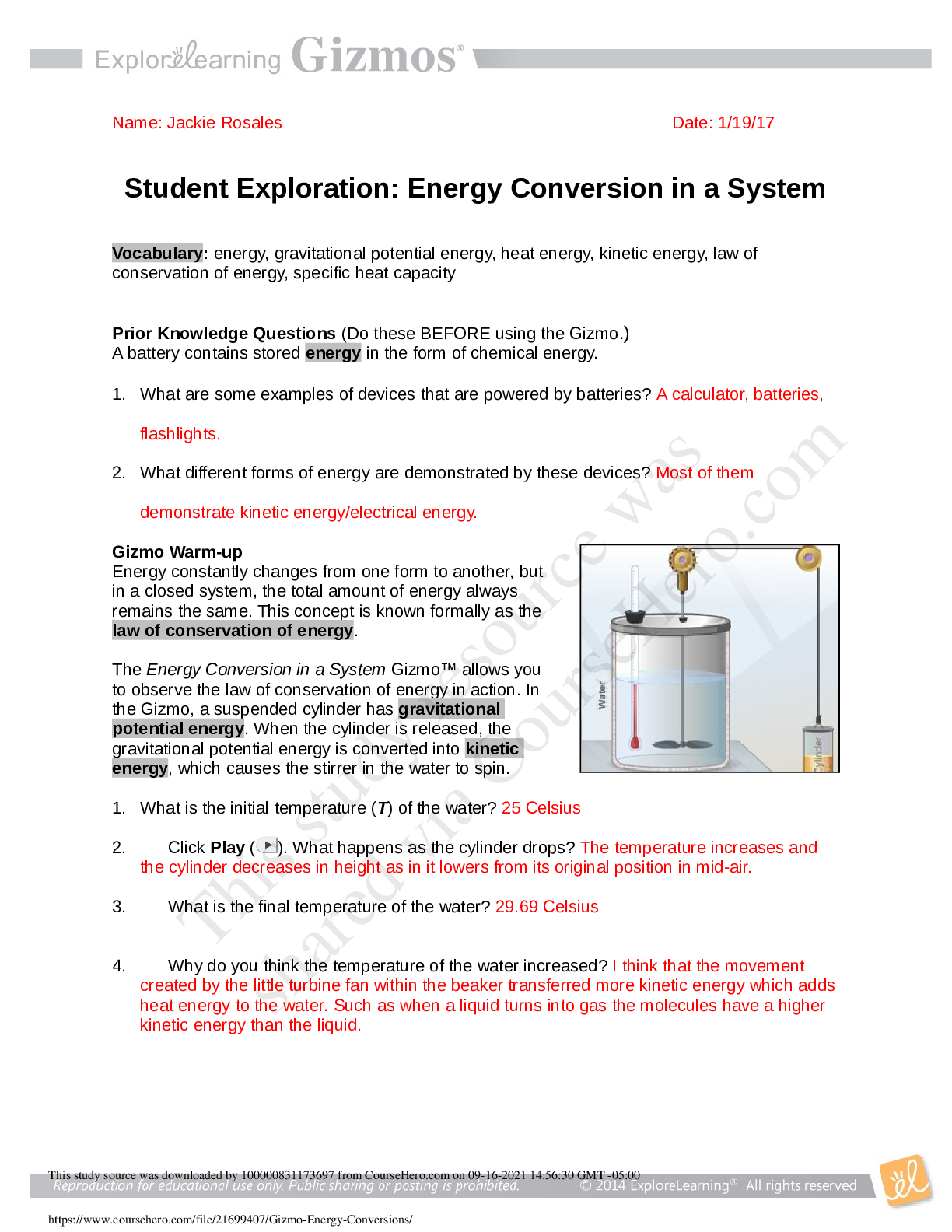
What is the initial temperature (T) of the water?
Click Play ( ). What happens as the cylinder drops?
What is the final temperature of the water?
Why do you think the temperature of the water increased?
Question: How does the cylinder’s initial height affect its gravitational potential energy?
1. Predict: How do you think increasing the cylinder’s height will affect the final temperature of
the water?
2. Gather data: Make sure the water’s Mass is 1.0 kg, its Temp is 25 °C, and the cylinder’s
Mass is 5 kg. Set the cylinder’s Height to 100 m. (Note: The large height scale used by the
Gizmo, while not practical in a real-world experiment, makes it easier to produce observable
temperature changes in the water.)
Click Play, and record the water’s final temperature in the table below. Repeat the
experiment at each cylinder height to complete the second column in the table.
3. Calculate: Subtract the water’s initial temperature from its final temperature to complete the
third column of the table.
An object’s GPE can be calculated by multiplying its height (h) by its mass (m) and
acceleration due to gravity (g): GPE = mgh. On Earth, g = 9.8 m/s2
. Calculate the cylinder’s
GPE for each of the trials you completed and fill in the last column of the table.
4. Analyze: Study the data you collected.
A. How does doubling the height of the cylinder affect its GPE?
B. How does doubling the cylinder’s GPE affect the change in temperature experienced
by the water?
Question: How does the cylinder’s mass affect its gravitational potential energy?
1. Predict: How do you think increasing the cylinder’s mass will affect the final temperature of
the water? Explain your prediction.
2. Gather data: Make sure the water’s Mass is still set to 1.0 kg and its Temp is 25 °C. Set the
cylinder’s Height to 500 m.
Use the Gizmo to complete the second column of the table below, and then calculate the
change in temperature and the cylinder’s GPE for each trial
Compare: Describe any patterns you see and compare your results with the results you got
when experimenting with the cylinder’s height in activity A:
Apply: Suppose the cylinder had a mass of 20 kg and started at a height of 2,000 m. If the
initial temperature of the water was 25 °C, what would be the final temperature? Explain.
Challenge: Not all substances heat up and cool down at the same rate. A substance’s
resistance to temperature change is described by its specific heat capacity, or specific
heat for short. For example, the specific heat of iron is 0.46 J/g °C. That means it takes 0.46
joules of heat energy to increase the temperature of a gram of iron by one degree Celsius.
Specific heat capacity can be calculated using the following equation: q = mc∆T.
In the equation, q represents the amount of heat energy gained or lost (in joules), m is the
mass of the substance (in grams), c is the specific heat capacity of the substance (in
J/g °C), and ∆T is the temperature change of the substance (in °C).
Click Reset. Set the water Mass to 1.0 kg (1,000 g). The cylinder should have a Mass of
5.0 kg and a Height of 500 m.
A. What is the gravitational potential energy of the cylinder? 24,500 J
B. If no energy is lost, how much heat energy is added to the water?500 kJ
C. What is the mass of the water? 1kg
D. What is the temperature change of the water? 5.86 J
E. What is the specific heat of the water? (Show your work below.) 24529.96 J/Kg C
F. How does the specific heat of water compare to the specific heat of iron?
Read More