Chapter 2 - Atoms, Molecules, and Ions
1. Which of the following is/are postulates of Dalton’s atomic theory?
1. Atoms combine in fixed ratios of whole numbers.
2. Atoms of each element have different properties.
3. Elements occur as solids, liquids, or gases.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1, 2, and 3
ANS: D PTS: 1 DIF: easy REF: 2.1
OBJ: List the postulates of atomic theory.
TOP: early atomic theory | atomic theory of matter
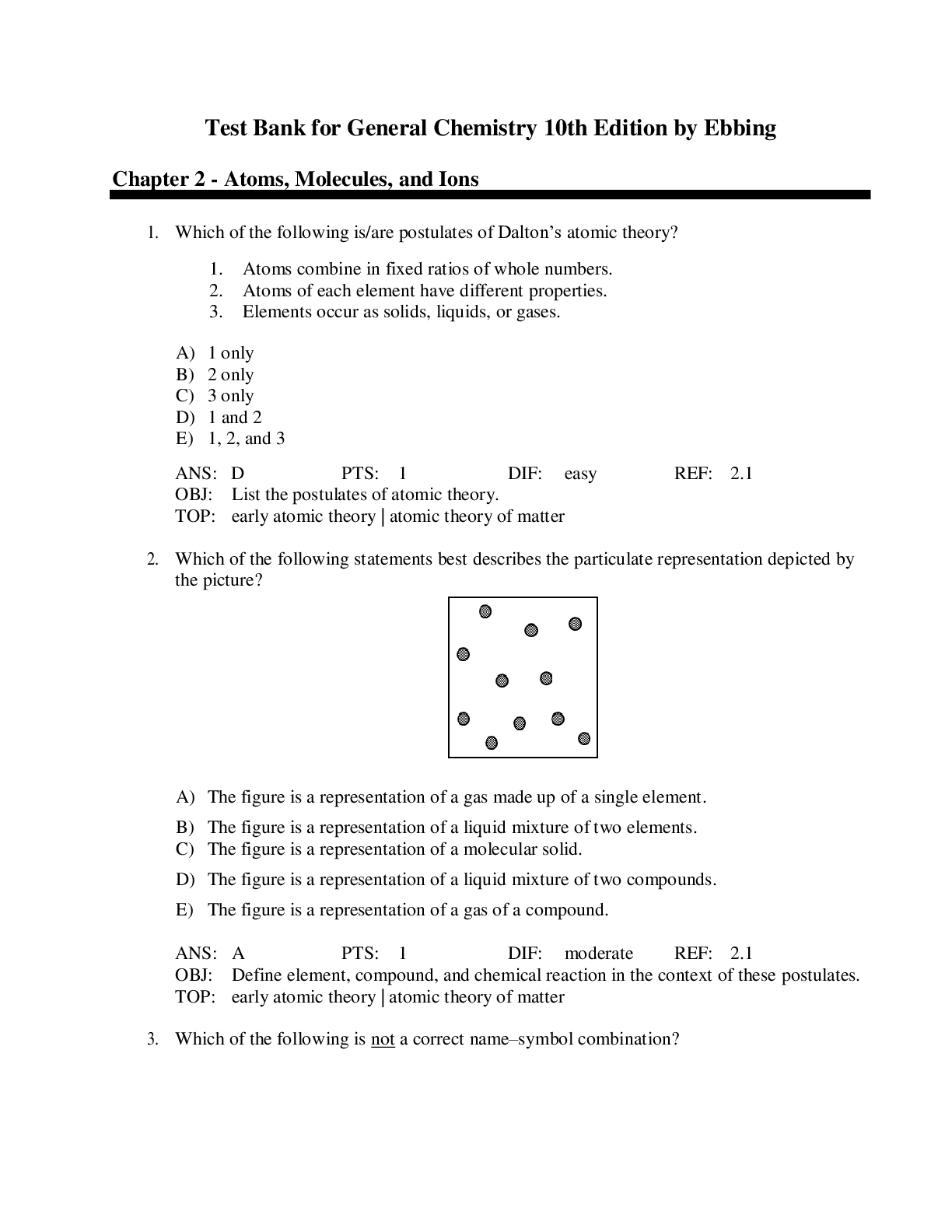
2. Which of the following statements best describes the particulate representation depicted by
the picture?
A) The figure is a representation of a gas made up of a single element.
B) The figure is a representation of a liquid mixture of two elements.
C) The figure is a representation of a molecular solid.
D) The figure is a representation of a liquid mixture of two compounds.
E) The figure is a representation of a gas of a compound.
ANS: A PTS: 1 DIF: moderate REF: 2.1
OBJ: Define element, compound, and chemical reaction in the context of these postulates.
TOP: early atomic theory | atomic theory of matter
3. Which of the following is not a correct name–symbol combination?
A) cobalt, Co
B) vanadium, V
C) neon, Ne
D) scandium, Sc
E) titanium, Mg
ANS: E PTS: 1 DIF: easy REF: 2.1
OBJ: Recognize the atomic symbols of the elements.
TOP: early atomic theory | atomic theory of matter
4. The symbol for tin is
A) T.
B) Tn.
C) Si.
D) Ti.
E) Sn.
ANS: E PTS: 1 DIF: easy REF: 2.1
OBJ: Recognize the atomic symbols of the elements.
TOP: early atomic theory | atomic theory of matter KEY:
atomic symbol
MSC: general chemistry
5. What is the symbol for the element phosphorus?
A) Po
B) P
C) Pt
D) K
E) Pr
ANS: B PTS: 1 DIF: easy REF: 2.1
OBJ: Recognize the atomic symbols of the elements.
TOP: early atomic theory | atomic theory of matter KEY:
atomic symbol
MSC: general chemistry
6. Which one of the following lists gives the correct symbols for the elements phosphorus,
potassium, silver, chlorine, and sulfur?
A) P, Po, Ag, Cl, S
B) K, Ag, Po, Cl, S
C) P, K, Ag, Cl, S
D) Ph, K, Ag, S, Cl
E) Ph, Po, Ag, Cl, S
ANS: C PTS: 1 DIF: easy REF: 2.1
OBJ: Recognize the atomic symbols of the elements.
TOP: early atomic theory | atomic theory of matter KEY:
atomic symbol
MSC: general chemistry
7. Which of the following lists gives the atomic symbols for potassium, magnesium, beryllium,
and sodium?
A) Po, Mn, Br, Na
B) P, Mn, Be, Se
C) K, Mg, Be, Na
D) Pt, Mg, Be, Sc
E) K, Mn, Br, Na
ANS: C PTS: 1 DIF: easy REF: 2.1
OBJ: Recognize the atomic symbols of the elements.
TOP: early atomic theory | atomic theory of matter KEY:
atomic symbol
MSC: general chemistry
8. The names of the elements whose symbols are Si, P, Mn, and S are, respectively,
A) silicon, phosphorus, manganese, and sulfur.
B) silicon, potassium, magnesium, and sulfur.
C) silver, phosphorus, magnesium, and sodium.
D) silver, potassium, manganese, and sodium.
E) silicon, potassium, manganese, and sulfur.
ANS: A PTS: 1 DIF: easy REF: 2.1
OBJ: Recognize the atomic symbols of the elements.
TOP: early atomic theory | atomic theory of matter KEY:
atomic symbol
MSC: general chemistry
9. Which of the following is the atomic symbol for the element cobalt?
A) CO
B) Co
C) C
D) co
E) All of the above
ANS: B PTS: 1 DIF: easy REF: 2.1
OBJ: Recognize the atomic symbols of the elements.
TOP: early atomic theory | atomic theory of matter KEY:
atomic symbol
MSC: general chemistry
10. A series of silicon–hydrogen compounds with the general formula SinH2n+2 can be
represented by the known compounds SiH4, Si2H6, and Si3H8. This best illustrates the law
of
A) multiple proportions.
B) conservation of charge.
C) definite composition.
Read More